Grand Challenges in Marine Biogeochemistry
Edited By Isabel Ferrera
The ocean plays a central role in our earth’s climate system and also provides a range of important ecosystem services, including food, energy, transport, and nutrient cycling. Marine biogeochemistry focuses on the study of complex biological, chemical, and physical processes involved in the cycling of key chemical elements within the ocean, and between the ocean and the seafloor, land and atmosphere. The ocean is increasingly perturbed by human induced alterations to our planet, including anthropogenic emissions of nitrogen, phosphorus, carbon and trace elements, and climate change. The establishment of a detailed understanding of biogeochemical processes, including their rates, is essential to the identification and assessment of climatic and chemical feedbacks associated with changes in the chemical and physical environment that are mediated through ocean biology, chemistry and physics. Important research areas in marine biogeochemistry involve the cycling of organic and inorganic forms of carbon, nitrogen and phosphorus, the cycling and biological roles of essential trace elements, and the fate and climatic impact of marine produced trace gases.
Biogeochemistry of Wetlands
Science and Applications
By K. Ramesh Reddy, Ronald D. DeLaune, Patrick W. Inglett
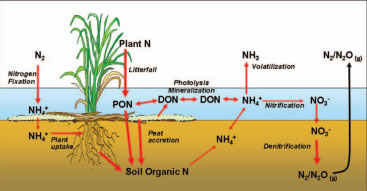
The globally important nature of wetland ecosystems has led to their increased protection and restoration as well as their use in engineered systems. Underpinning the beneficial functions of wetlands are a unique suite of physical, chemical, and biological processes that regulate elemental cycling in soils and the water column. This book provides an in-depth coverage of these wetland biogeochemical processes related to the cycling of macroelements including carbon, nitrogen, phosphorus, and sulfur, secondary and trace elements, and toxic organic compounds.
In this synthesis, the authors combine more than 100 years of experience studying wetlands and biogeochemistry to look inside the black box of elemental transformations in wetland ecosystems. This new edition is updated throughout to include more topics and provide an integrated view of the coupled nature of biogeochemical cycles in wetland systems. The influence of the elemental cycles is discussed at a range of scales in the context of environmental change including climate, sea level rise, and water quality. Frequent examples of key methods and major case studies are also included to help the reader extend the basic theories for application in their own system.
Formation of aliphatic carbon–nitrogen bonds
James M. Coxon, Juliet A. Gerrard, Sir Richard Norman, James M. Coxon
Carbon–nitrogen bonds are most commonly formed when nucleophilic nitrogen reacts with electrophilic carbon; less commonly, they are formed by reaction of electrophilic nitrogen with nucleophilic carbon. The lone electron pair of primary, secondary and tertiary amines can displace halide from a primary halide. Synthesis using this methodology is complicated by further reactions of a product primary, secondary or tertiary amine with further primary halide. Ammonia reacts with aldehydes but often the products are unstable unless the aldehyde carbon is attached to a strongly electron-attracting group. Acetone, however, behaves differently, first undergoing self-condensation under the influence of ammonia, which acts as a base rather than as a nucleophile, the carbonyl carbon being somewhat more hindered than in an aldehyde and less electropositive. The ester carbonyl carbon with an adjacent electron-rich ether oxygen is less reactive to nucleophilic attack than the ketone group.
Mercury biogeochemistry over the Tibetan Plateau: An overview
Published in Critical Reviews in Environmental Science and Technology
Ruiyang Sun, Guangyi Sun, Sae Yun Kwon, Xinbin Feng, Shichang Kang, Qianggong Zhang, Jie Huang, Runsheng Yin
Here, we reviewed the available literature to obtain a comprehensive understanding of Hg biogeochemistry over the TP. The biogeochemical Hg cycling is characterized by the following features: (1) There are existing but limited local emission sources of anthropogenic Hg in the TP. The Indian Summer Monsoon is an important transporter of atmospheric Hg pollution into the inland TP; (2) “Cold trapping effect” plays an important role in the atmospheric Hg deposition over the TP. Glacier, vegetation, and soil act as important “sinks” of atmospheric Hg pollution; (3) Enhanced anthropogenic activities around the TP, climate warming and glacier melting have the potential impacts to affect the behavior and distribution of Hg; (4) Significant bioaccumulation of MeHg (>100 ng/g) has been found in the Tibetan aquatic food chains. Considering that transboundary transport is responsible for the widespread Hg pollution in the TP, international/regional collaborations regarding Hg emission regulations are needed to reduce the migration of Hg and to mitigate adverse Hg pollution impacts on the TP.
Radioiodine Biogeochemistry and Prevalence in Groundwater
Published in Critical Reviews in Environmental Science and Technology
D. I. Kaplan, M. E. Denham, S. Zhang, C. Yeager, C. Xu, K. A. Schwehr, H. P. Li, Y. F. Ho, D. Wellman, P. H. Santschi
These recent studies have led to a more mechanistic understanding of radioiodine biogeochemistry. The objective of this review is to describe these advances and to provide a state of the science of radioiodine biogeochemistry relevant to its fate and transport in the terrestrial environment and provide information useful for making decisions regarding the stewardship and remediation of 129I contaminated sites. As part of this review, knowledge gaps were identified that would significantly advance the goals of basic and applied research programs for accelerating 129I environmental remediation and reducing uncertainty associated with disposal of 129I waste. Together the information gained from addressing these knowledge gaps will not alter the observation that 129I is primarily mobile, but it will likely permit demonstration that the entire 129I pool in the source term is not moving at the same rate and some may be tightly bound to the sediment, thereby smearing the modeled 129I peak and reducing maximum calculated risk.
Biogeochemistry of Weathering Processes in Monuments
Published in Geomicrobiology Journal
In this review some case studies are presented to illustrate the complex interactions between organisms, substratum, and environment as an indication of the biogeochemical processes originating in monuments subjected to different environmental conditions. Building materials in urban environments develop black crusts, composed mainly of gypsum and polycyclic aromatic hydrocarbons. Gypsum is used by cyanobacteria as a sulfate source, whereas heterotrophic bacteria are able to use polycyclic aromatic hydrocarbons as a sole carbon and energy source. On the other hand, in hypogean environments with natural openings to sunlight, calcifying cyanobacteria mobilize calcium from the substratum to produce calcite crystals, which are deposited on the sheath and form a mineral coat. In rural environments, most conspicuous biogeochemical processes derive from natural colonization of building materials by cyanobacteria, algae, and lichens. Although cyanobacteria and algae dissolve calcium carbonate, lichens in some cases confer protection through the formation of oxalate patina, which protects the surfaces from weathering.
Organic carbon biogeochemistry of Lake Superior
Published in Aquatic Ecosystem Health & Management
James B. Cotner, Bopaiah A. Biddanda, Wataru Makino, Edward Stets
We examined the organic carbon budget for the Earth’s largest lake, Lake Superior, in the Laurentian Great Lakes. This is a unique, ultra-oligotrophic system with many features similar to the oligotrophic oceanic gyres, such as dominance of microbial biomass and dissolved organic carbon in biogeochemical processes. Photo-autotrophy is the dominant source of reduced organic matter in the lake. Areal rates of primary production are among the lowest measured in any aquatic system, and are likely a result of cold water temperatures and low nutrient concentrations in the lake. Allochthonous riverine organic carbon inputs were estimated at about 10 percent of photo-autotrophic production. Atmospheric carbon deposition has not been measured to any significant extent but we estimate it at 0.16 to 0.41 Tg yr−1 . All together, allochthonous carbon sources provide 13 to 19 percent of photo-autotrophic production.
 Africa
Africa  China
China  Japan
Japan