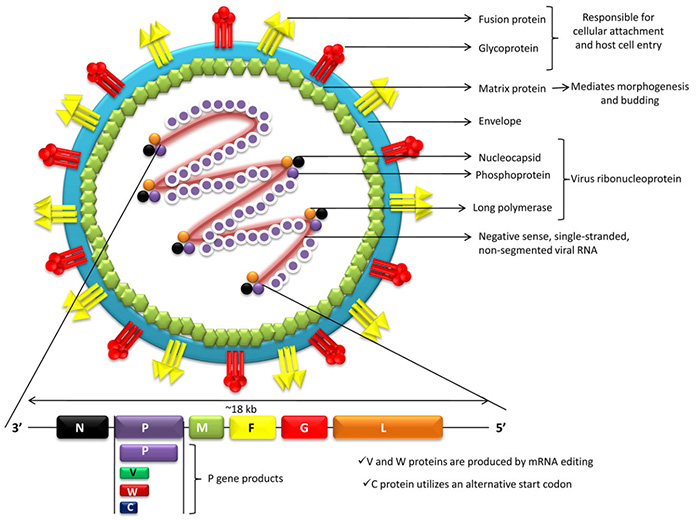
Order Mononegavirales
Paul Pumpens, Peter Pushko, Philippe Le Mercier
The chapter will describe the VLPs derived from the order Mononegavirales that is the second largest among other negative single-stranded RNA virus orders, after the order Bunyavirales, by number of families, currently 11. The story involves VLPs of such well-known pathogens as Borna disease virus of the Mammalian 1 orthobornavirus species from the Orthobornavirus genus, family Bornaviridae; members of the Ebolavirus and Marburgvirus genera, family Filoviridae; measles virus and peste-des-petits-ruminants virus (PPRV) of the Morbillivirus genus, human parainfluenza viruses 2 and 4 and mumps virus of the Orthorubulavirus genus, Menangle and Tioman viruses of the Pararubulavirus genus, Nipah virus of the Henipavirus genus, Newcastle disease virus of the Avian orthoavulavirus 1 species from the Orthoavulavirus genus, and Sendai virus, as well as human parainfluenza viruses 1 and 3 of the Respirovirus genus, all belonging to the family Paramyxoviridae; human respiratory syncytial virus (hRSV) of the Human orthopneumovirus species from the Orthopneumovirus genus, and human metapneumovirus of the genus Metapneumovirus, family Pneumoviridae; rabies virus of the Lyssavirus genus and vesicular stomatitis virus (VSV) of the Indiana vesiculovirus species from the Vesiculovirus genus, family Rhabdoviridae.
Porcine Rubulavirus (Blue Eye Disease Virus)
Dongyou Liu
The blue eye disease, affecting pigs of all ages, is caused by a virus of the Rubulavirus genus, Paramyxoviridae family [1]. The rst cases were reported in 1980, in hog farms at La Piedad, Michoacán, Mexico. Clinical signs, mostly of nervous nature, were observed in piglets younger than 30 days. New outbursts were reported later on the same year in the nearby states of Jalisco and Guanajuato and in 1983 in Mexico City, Nuevo León, Hidalgo, Tlaxcala, Tamaulipas, Puebla, Campeche, and Querétaro. Since its emergence, the disease has been difcult to control, and by 1992, it had spread to 16 states in Mexico [2]. According to a seroprevalence survey of porcine rubulavirus (PRV) performed in 2004, a prevalence of 10%–30% was found in 18 Mexican states [3].
Newcastle disease (ND)
Todd Applegate
ND is one of the most devastating diseases affecting the poultry industry worldwide (Ganar et al., 2014). It is caused by the highly contagious ND virus (NDV), which is a member of the genus Avulavirus (also known as APMV-1, avian paramyxovirus serotype-1) in the family Paramyxoviridae. They are an extremely diverse group of viruses, and, based on disease severity, NDV strains are classified into velogenic (highly virulent), mesogenic (moderately virulent), lentogenic (mildly virulent) and asymptomatic pathotypes (Alexander et al., 2012). The clinical signs and pathology of ND can range from inapparent infections to severe disease with high mortality, depending on the virus strains and the susceptibility of the host species. Around 250 bird species have been reported to be susceptible to NDV, and virus infections in feral and pet birds can influence virus spread and initiation of major outbreaks and panzootics in poultry. ND is a major global threat to the poultry industry as it has been reported in most countries. While it is endemic in majority of the countries, including some of largest poultryproducing nations, several countries maintain disease-free status despite the accidental incursions of the virus. In NDV-free countries, statutory surveillance measures are in place and outbreaks of NDV are notifiable to the OIE (Office International des Epizooties) and are associated with considerable economic impact including international trade restrictions.
The structure of the nucleoprotein binding domain of lyssavirus phosphoprotein reveals a structural relationship between the N - RNA binding domains of Rhabdoviridae and Paramyxoviridae
Published in RNA Biology
Olivier Delmas, Rene Assenberg, Jonathan M Grimes, Hervé Bourhy
The phosphoprotein P of non-segmented negative-sense RNA viruses is an essential component of the replication and transcription complex and acts as a co-factor for the viral RNA-dependent RNA polymerase. P recruits the viral polymerase to the nucleoprotein-bound viral RNA (N-RNA) via an interaction between its C-terminal domain and the N-RNA complex. We have obtained the structure of the C-terminal domain of P of Mokola virus (MOKV), a lyssavirus that belongs to the Rhabdoviridae family and mapped at the amino acid level the crucial positions involved in interaction with N and in the formation of the viral replication complex. Comparison of the N-RNA binding domains of P solved to date suggests that the N-RNA binding domains are structurally conserved among paramyxoviruses and rhabdoviruses in spite of low sequence conservation. We also review the numerous other functions of this domain and more generally of the phosphoprotein.
Neonatal Syncytial Giant Cell Hepatitis with Paramyxoviral-like Inclusions
Published in Ultrastructural Pathology
Syncytial giant cell hepatitis in the neonatal period has been associated with many different etiologic agents and may present initially as cholestasis. Infectious causes are most common and include: (1) generalized bacterial sepsis, (2) viral agents, (3) toxoplasmosis, (4) syphilis, (5) listeriosis, and (6) tuberculosis. Viral hepatitis may be due to cytomegalovirus, rubella virus, herpes simplex, HHV-6, varicella, coxsackievirus, echovirus, reovirus 3, parvovirus B19, HIV, enteroviruses, paramyxovirus, and hepatitis A, B, or C (rare). Giant cell hepatitis may result in fulminant liver failure with massive hepatocyte necrosis and severe liver dysfunction leading to death, resolution with severely compromised liver function, or liver transplantation. The authors report a 6-week-old male who had an unremarkable perinatal period, became jaundiced after developing diarrhea, and subsequently developed liver dysfunction with massively increased liver enzymes and a coagulopathy. Open wedge and core liver biopsies were performed to determine if the patient should be listed for liver transplantation. Giant cell hepatitis with a significant mixed lymphocytic and neutrophilic infiltrate was present on both the wedge and core biopsies. The residual 60% of hepatocytes had ballooning degeneration and many possessed pyknotic nuclei. The hepatocytes were arranged in a pseudoacinar pattern. Electron microscopy showed paramyxoviral-like inclusions in the giant cells, characterized as large inclusions with fine filamentous, beaded substructures (18-20 nm). Paramyxoviridae are nonsegmented, negative-sense, single-stranded RNA viruses. This family is divided into the Paramyxovirinae subfamily containing respirovirus (Sendai virus, parainfluenza virus type 3), rubula virus (mumps, parainfluenza virus type 2), and morbillivirus genera (measles); and Pneumovirinae subfamily (pneumovirus genus [respiratory syncytial virus]). Supportive care to determine if hepatic function resolves following the viral episode, liver transplantation with fulminant liver failure, and ongoing evaluation in those who recover to assess chronic liver disease are necessary. Ultrastructural evaluation may unmask the etiologic agent for hepatitis and direct therapy.
 Africa
Africa  China
China  Japan
Japan